Implementing CRISPR/Cas9 mutations in zebrafish
A new study is implementing CRISPR/Cas9 mutations in zebrafish (Danio rerio) in order to be able to investigate the influence of different genes on the gut flora. The zebrafish is a great study organism for both illnesses as well as biological mechanisms due to its short generation time.
New knowledge about our gut flora
The fact that a healthy gut flora is important for our health is well known and in recent years there has been a growing focus on the influence of our food and lifestyle on our gut flora. However, new research suggest that our genes also play an important role – but how? That is one of the questions that the researchers at the new Center for Evolutionary Hologenomics are asking.
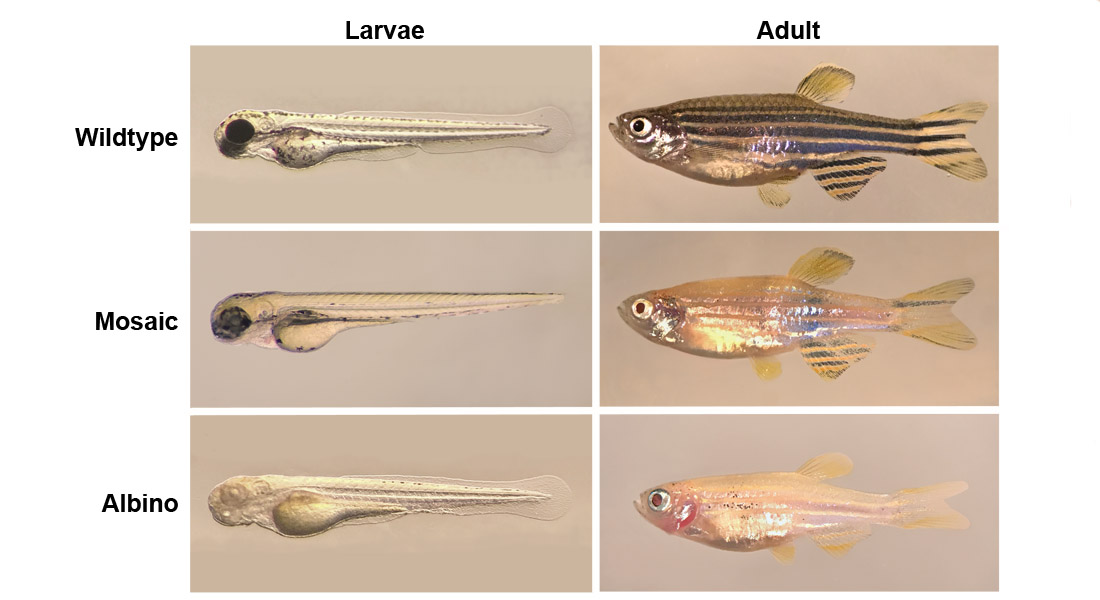
Researchers at GLOBE Institute and the Department of Veterinary and Animal Sciences are collaborating to solve this question by implementing the CRISPR/Cas9 mutation technology in zebrafish. They have started by knocking out the gene responsible for pigmentation in zebrafish and as a result they have created albino fish.
The CRISPR/Cas9 technology can be combined with already exciting tools such as transgenic lines and thereby represents a simple way to create mutations in zebrafish in order to investigate certain biological functions. The cells and functions of the immune system is often the same in fish and humans, so despite the obvious differences these basic functions and immunological pathways and mechanisms can be investigated in zebrafish and be applied for research on other mammals.
How to create albino fish
The researchers only have 30 minutes to inject the reaction mixture – a mix of guide RNA and Cas9 protein – into the yolk sac of a newly laid egg so that the mixture can reach the egg on the first cell stage. Inside the egg the guide RNA will guide the Cas9 protein to the exact position on the DNA where Cas9 nicks the double stranded DNA and damage the gene of interest. After two days a phenotypic mutation will show in the embryos. Some mutation will not be visible with the naked eye, which is why the gene sequences should be analysed. In order to identify the best adult fish for breeding pure lines the genes should be analysed in more fish and the best ones chosen. The aim of this technique is to be able to knock out genes which codes for proteins that affects the fish microbiome.
The albino fish are being used to investigate whether the pigment affects the microbiome on the surface of the fish and later on the researchers will knock out more genes that are thought to influence the gut microbiome. The first step is to knock out the gene that codes for the interferon regulatory factor 8 (Irf8), which a subpopulation of macrophages (a certain type of immune cells) is dependent on. This will lead to less macrophages in the gene manipulated fish. The researchers believe that this manipulation will affect the bacteria composition in the gut.
The microbiome in the gut of the fish is dependent of different factors and the manipulation of macrophages will most likely play a role. The results can increase our knowledge about how the genetic profile of the host fish influences its gut flora and provide knowledge on how human genes can affect our gut flora.
The zebrafish is a great model organism
The reason why zebrafish are great as model organisms is that their development is fast compared to for instance a laboratory mouse. Furthermore, creating genetic mutations in the fish is relatively simple as the embryonic development is external. Two days after fertilisation a fish larva has been developed with eyes, brain, liver and a heart and since the tiny fish larva is transparent all these organs can be monitored. Three months later the fish are ready to reproduce.
With a tool like the CRISPR/Cas9 technology new mutations can be introduced and pure breeds can be created with the wanted mutation. This is very useful especially for functional studies where you investigate responses in an organism with and without a protein which the gene is coding for. The CRISPR fish are made by Louise von Gersdorff Jørgensen and Moonika Haahr Marana, Section for Aquatic Pathobiology. The team is happy to collaborate with other researchers on projects involving zebrafish.
Contact:
The work is supported by the Independent Research Fund Denmark as well as the Danish National Research Foundation.
